Ion Magnesium carbonate Chemical compound Inorganic compound, information symbol, text, trademark png | PNGEgg
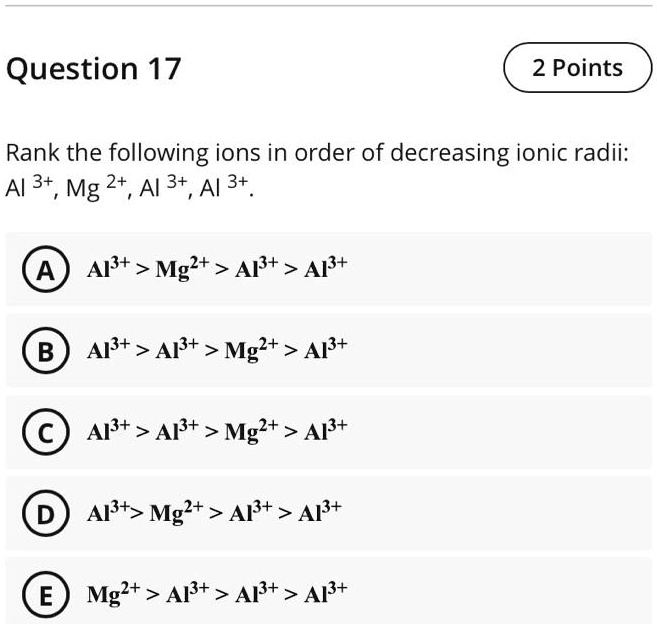
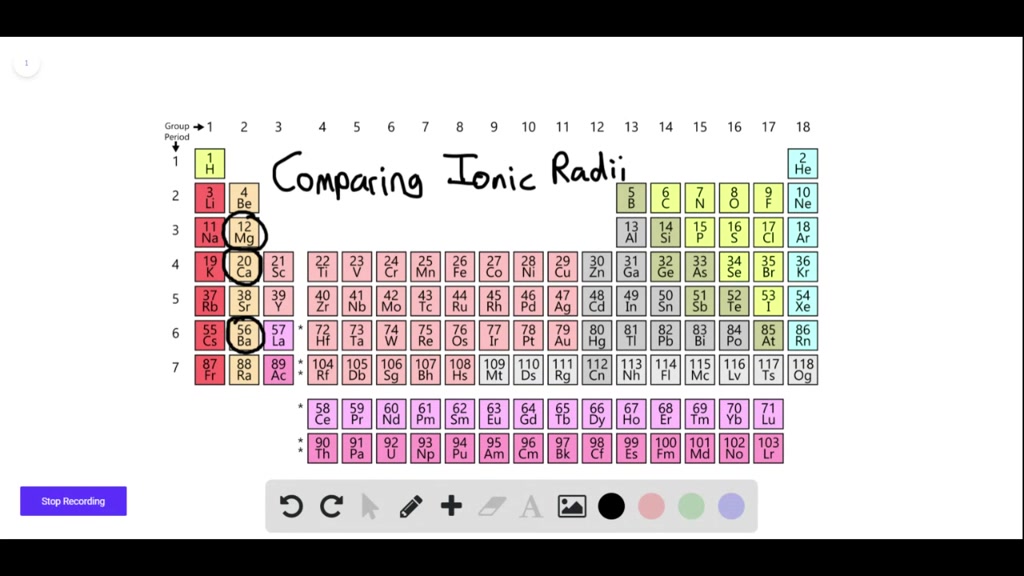
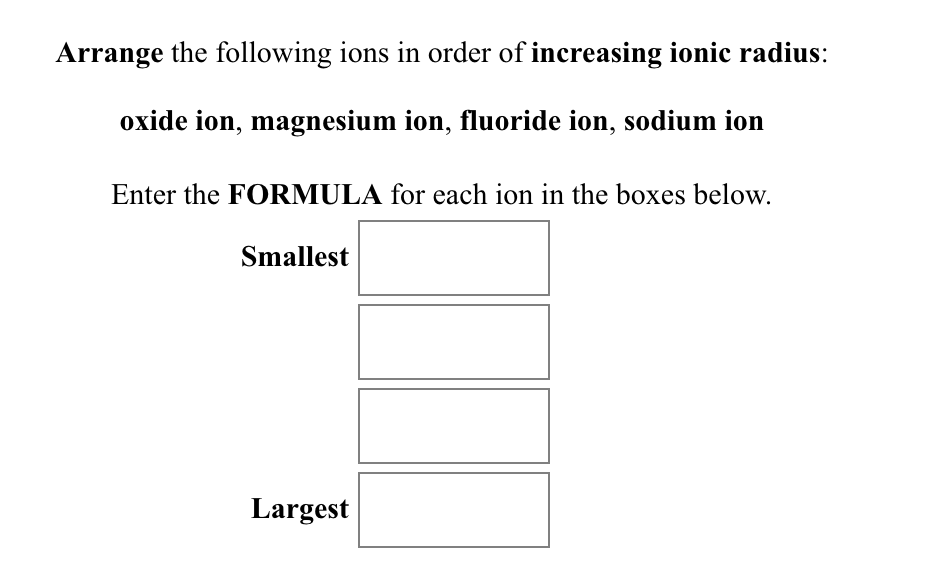
SOLVED: Question 17 2 Points Rank the following ions in order of decreasing ionic radii: Al 3+, Mg 2+, Al 3+,Al 3+ Al3+ > Mg2+ > Ap+ > Ap+ AP+ AP+ Mg2+ >

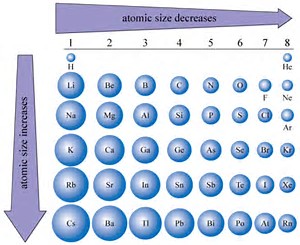
Comparison of ionic radius and weight among Li, Na, and K alkali metals. | Download Scientific Diagram